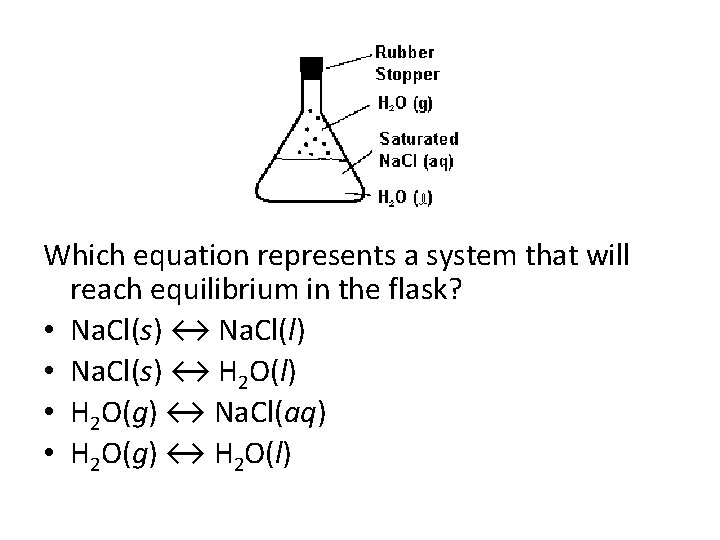
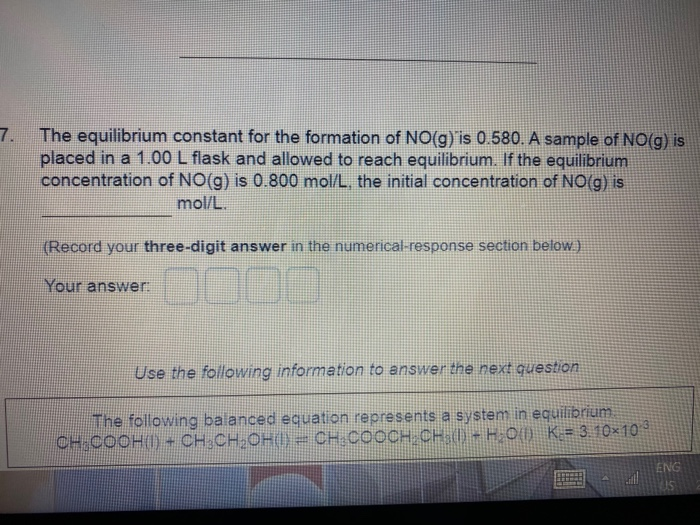
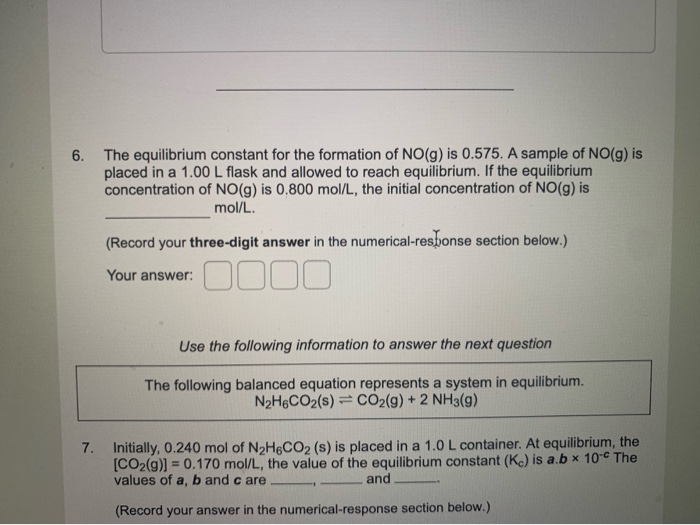
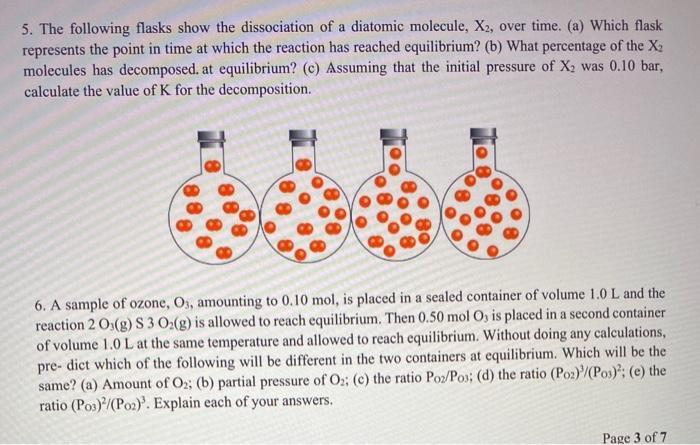
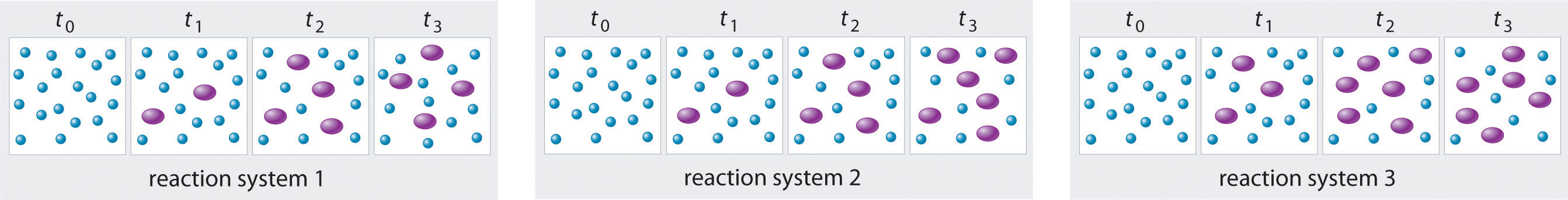
Which Equation Represents A System That Will Reach Equilibrium In The Flask
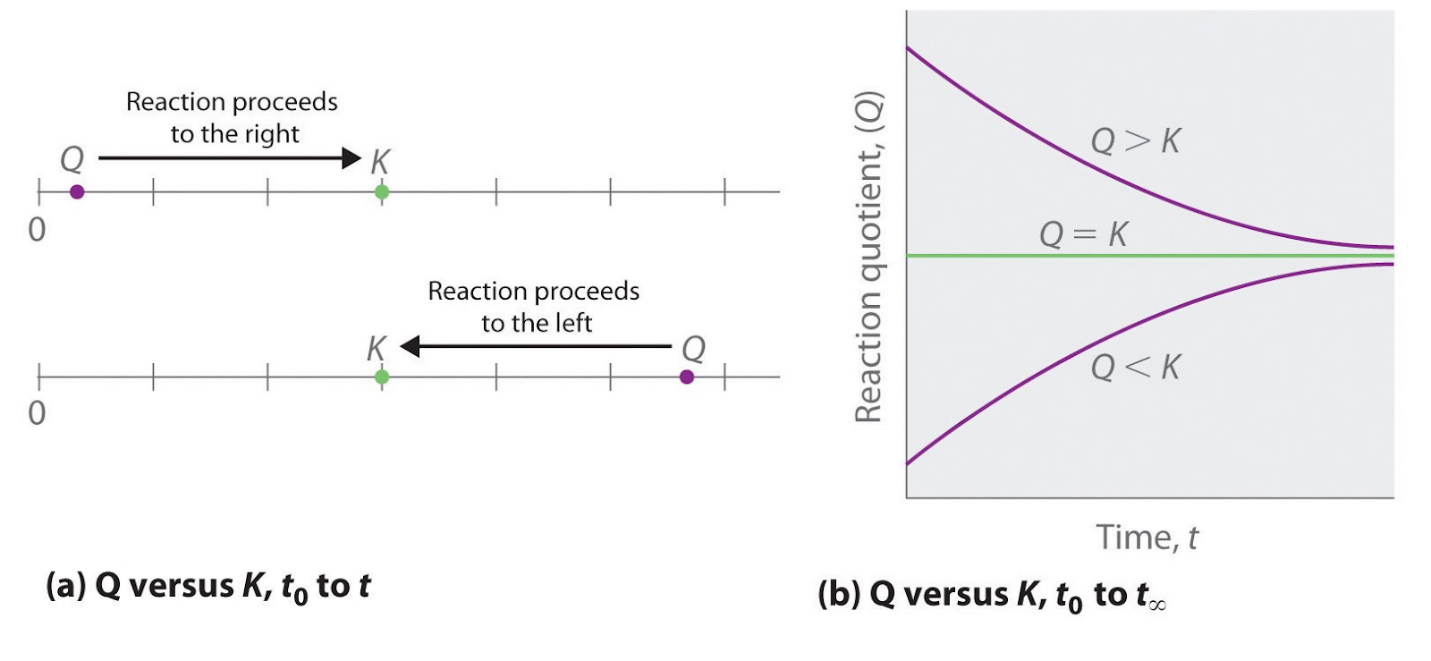
Which equation represents a system that will reach equilibrium in the flask. At equilibrium it is found that the concentration of FeSCN2 is 400 M. The equilibrium curve at 1 atm atmospheric pressure for methanol and water can be. The following equation represents the decomposition of a generic diatomic element in its standard state.
The purpose of the present paper is to study the performance of activated carbon in the water filtering system. The difference was more noticeable as the. Change in velocity acceleration time taken 200 ms 100 s.
The partition coefficient abbreviated P is defined as a particular ratio of the concentrations of a solute between the two solvents a biphase of liquid phases specifically for un-ionized solutes and the logarithm of the ratio is thus log P. Whenever an external pressure is applied to a confined fluid at rest either liquid or gas the pressure increases at every point in the fluid by the amount of the external pressure applied. Now calculate the change in velocity.
4 can be developed for the terminal velocity by rearranging Fb Fg Fd 0. Fill a small Erlenmeyer flask with water and several drops of food coloring and stopper the flask with a two-hole rubber stopper. B When a solution of sucrose with an initial concentration of 0150 M reaches equilibrium the concentration of sucrose is 165 10 7 M.
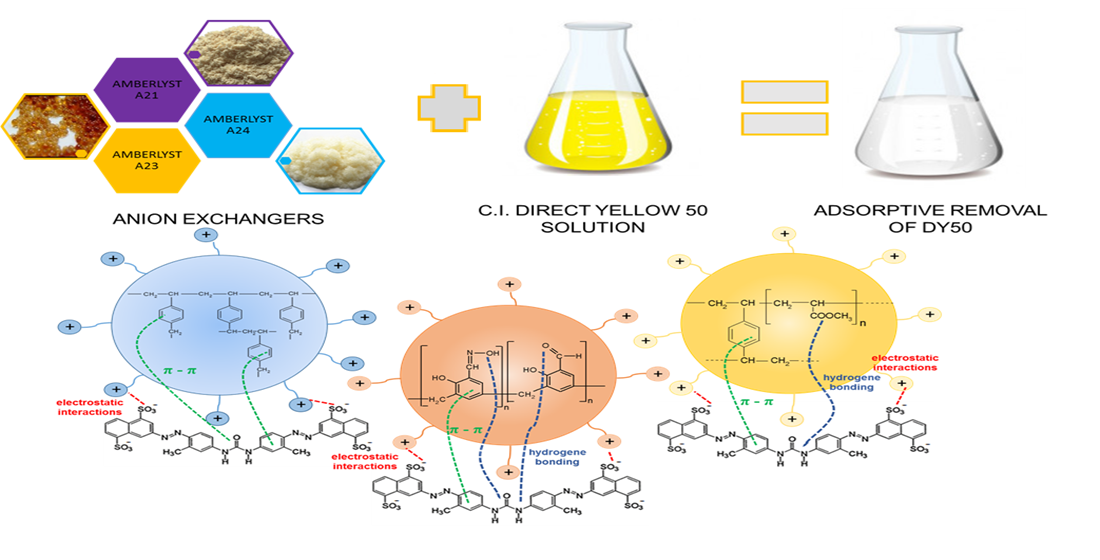
One atmosphere l atm. Therefore another equilibrium property is involved namely the amount of retained extract per kg of inert solids M. The time needed to reach equilibrium on the modified and unmodified ion exchangers was almost the same 60 min at the initial AsV concentrations 25 50 mgdm 3 and 120 min at 100 mgdm 3.
Q12 1 gm sample of KClO3 was heated under such conditions that a part of it decomposed according to the equation 1 2KClO3 2 KCl 3O2 and remaining underwent change according to the equation. 275ff When one of the solvents is water and the other is a non-polar solvent then the log P value is a measure of lipophilicity or hydrophobicity. What is the solubility product of barium fluoride if.
The velocity that brings the forces into equilibrium is called the terminal velocity or terminal settling velocity for the case when the particle is settling through the fluid. 12 X2 g X g Assume that the standard molar Gibbs energy of formation of X g is 514 kJ mol-1 at 2000 K and -5110 kJ mol-1 at 3000 K.
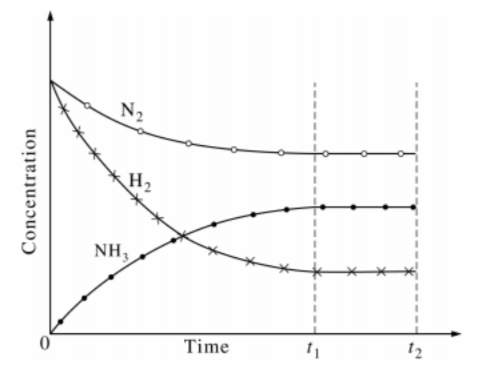
In an experiment 0030 mol NO_2 was introduced into a 200-L flask and the following reaction was allowed to come to equilibrium at 298 K.
12 X2 g X g Assume that the standard molar Gibbs energy of formation of X g is 514 kJ mol-1 at 2000 K and -5110 kJ mol-1 at 3000 K. Q12 1 gm sample of KClO3 was heated under such conditions that a part of it decomposed according to the equation 1 2KClO3 2 KCl 3O2 and remaining underwent change according to the equation. However it was found that the A33E-LaIII equilibrium sorption capacities q e were higher than those of A33E. A solution with initial concentrations of 600 M Fe3 and 100 M SCN1- is allowed to reach equilibrium. If at equilibrium the flask was found to contain 0078 M HI. Make a revision and communicate with your writer exactly what you want adjusted or improved on your paper. At equilibrium which concentrations will be greater than 10 moleL and which will be less than 10 moleL. Activated carbon is commonly used in water treatment to remove water contaminants. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
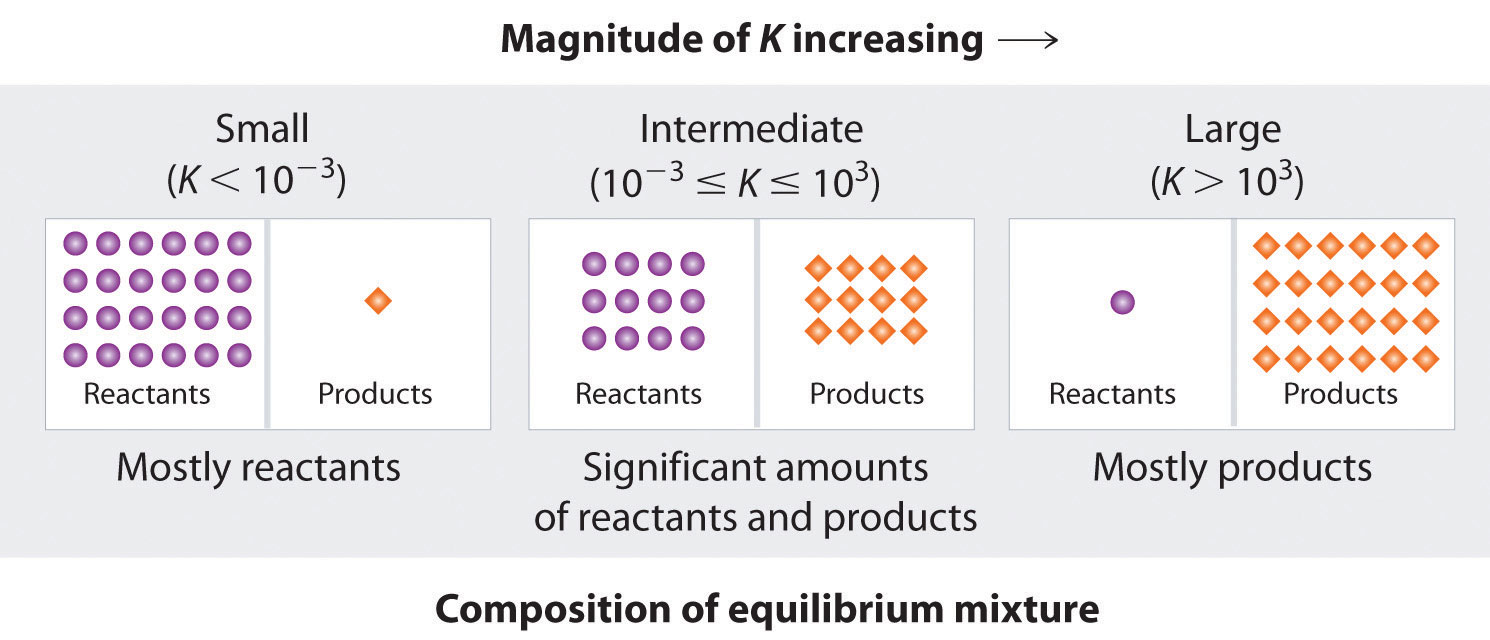
Now calculate the change in velocity. Determine the value of K the thermodynamic equilibrium constant at each temperature. Q12 1 gm sample of KClO3 was heated under such conditions that a part of it decomposed according to the equation 1 2KClO3 2 KCl 3O2 and remaining underwent change according to the equation. Three solid substances x y and z are heated the number of free surfaces of substances x and y decreases to zero and one respectively. Therefore another equilibrium property is involved namely the amount of retained extract per kg of inert solids M. A 2-L flask is filled with 02 mol of HI. One atmosphere l atm.



















Post a Comment for "Which Equation Represents A System That Will Reach Equilibrium In The Flask"